Influence of diatomaceous earth on the material properties of clay bricks
An increasing content of kieselguhr was added to a typical, marly clay used for the production of lightweight clay bricks, and the resulting formulations were processed to laboratory bricks. The addition of 20 wt% kieselguhr effects a 16.5 % reduction in the apparent density and a 15.4 % reduction in the body thermal conductivity λ10,tr., with a simultaneous increase in compressive strength by 10.9 %. A maximum increase in compressive strength by 27.7 % is obtained with an addition of 10 wt% kieselguhr. During ceramic firing, the high-strength diatom scaffolds consisting of amorphous silicic acid sinters together with the matrix of the ceramic body, leading to an increase in its compressive strength, with a simultaneous reduction in its apparent density and the body thermal conductivity. Tests with increasing sintering temperatures show that the structure of diatoms is preserved during the firing process.
Introduction
Successful building materials for the future should be characterized by a multitude of favourable material characteristics. One of the most important requirements is excellent thermal insulation, which can be achieved with a minimized equivalent thermal conductivity of the wall building material. Moreover, wall building materials should demonstrate chemically inert behaviour, be incombustible and diffusible as well as feature high compressive strengths [1, 2].
In order to minimize the thermal conductivity of high-thermal-insulation vertically perforated clay bricks, for many years the clay brick industry has been very successfully applying the methods described below to lower heat transfer in vertically perforated clay blocks:
Reduction of the body thermal conductivity by means of porosification
Lengthening of the heat flow paths based on optimization of the perforation pattern and
Filling of the cavities in the vertically perforated clay bricks to exclude heat transfer by radiation and convection
Body porosification can be obtained with the addition of organic and/or inorganic poreformers to the body formulation. With the pore formation in heavy clay ceramics, a reduction in the body thermal conductivity is achieved. However, the compressive strength of the body also suffers as the result of this process [3]. The only known exception so far is pore formation with kieselguhr. Here, the body apparent density and the body thermal conductivity λ10,tr. are lowered with increasing compressive strength. This effect has been termed the “ceramic paradox” [4-8].
Objective
In this work, first the effect of the “ceramic paradox” is to be verified on a typical marly masonry brick clay based on a continuous increase in the addition of kieselguhr. The laboratory bricks prepared are fired at a maximum sintering temperature of 900 °C and then the material characteristics. i.e. apparent density, body apparent density, true density, porosity, body thermal conductivity, compressive strength, and modulus of elasticity, are determined and compared with the results for brick reference specimens prepared without the addition of kieselguhr. In the further course of the work, we want to ascertain whether the diatoms incorporated in the brick matrix survive the temperatures between 900 °C to 1000 °C without any change to their structure. For this purpose, clay formulations containing kieselguhr are exposed to temperatures rising from room temperature to 1 500 °C by means of a hot stage microscope and simultaneous thermal analysis (STA).
Kieselguhr
Kieselguhr or diatomaceous earth consists of sedimented shells of single-cell diatoms, a type of microalgae. Fossilized diatom sediment originates from the Tertiary, Quaternary, Eocence and Miocene and are around 1 to 50 million years old [9-12]. Deposits are distributed around the world and the material is extracted in surface mining. In Europe, kieselguhr is found in deposits in Bavaria (Neuburg on the Danube), in the Lüneburger Heide (Neuohe) and in Denmark (island of Fur). From the kieselguhr-containing “Molererde”, in Denmark, so-called “Moler bricks” are produced, which are used in kiln insulation [13]. Several thousand species of diatoms are known, which differ in shape and size. There are, for instance, tubular, oval- and disc-shaped species with dimensions from around 5 to 400 µm. The shells of the diatoms consist practically completely of amorphous silicic acid (SiO2); their morphology is of non-crystalline structure. It can be concluded from this that their thermal conductivity is lower than that of a crystalline SiO2 structure (quartzite). The cavities inside the diatoms are enclosed by complex-structured shells consisting of amorphous silicic acid, which on account of their tectonic, sheath-like structure have extremely high inherent stabilities with minimum density. The shell structures of the diatoms are “minimalistic biological structures“, that means that with minimum energetic input and material (SiO2), the diatoms have realized a high degree of static strength in their shell structures. As a result of the small dimensions of the diatoms on micrometre scale and the pore space inside the diatoms, the addition of the kieselguhr leads to microporosity of the brick body. Because of sintering of the high-strength amorphous diatoms shells together with the body matrix (» Figs. 1-3), the compressive strength of the bricks increases. If the pore space, which measures only a few micrometres, inside the sintered diatoms can be preserved, it is possible to lower the apparent and body apparent density as well as the body thermal conductivity of the brick ceramic [4-8]. As pore formation with kieselguhr leads to inorganic microporosity, its impact on the body apparent density and body thermal conductivity of brick ceramic cannot be in the same order of magnitude as macropore formation with sawdust and polystyrene.
Experimental
For the preparation of clay formulations, increasing mass percentages of kieselguhr (5, 10, 15 and 20 wt%) were added to a typical marly clay formulation for the production of vertically perforated lightweight clay bricks. The kieselguhr used here was purchased from a well-known supplier. To investigate exclusively the effects of kieselguhr addition on the brick properties, no other poreformers were added. To exclude the influence of cutting and yield point textures during the extrusion process, the clay bricks were not formed by means of an extruder, but with a hydraulic parallel press instead (» Fig. 4).
The green bricks still wet after shaping on the press were dried first for 24 h in room air and then for another 24 h in a drying cabinet at a temperature of 40 °C. Then the green bricks were finally dried for another 24 h at 105 °C. The firing process was conducted in a laboratory oven with electric resistance heating, the dried green bricks being heated over 10 h from room temperature to 900 °C before this final firing temperature was held constant for another 5 h. After the oven had been switched off, it cooled slowly in an unregulated process over a period of around 24 h down to room temperature.
The fired laboratory bricks were sawn with a brick saw and ground plane-parallel with a surface grinding machine to the exact dimensions (L x B x H) 10.0 cm x 10.0 cm x 3.0 cm. Because the two machining steps are performed with water rinsing, the wet laboratory bricks were then redried for 24 h at 40 °C and for another 24 h at 105 °C (» Fig 5). The dried laboratory bricks were stored at room temperature in a dessicator until they were used for determination of the specific material characteristics [6-8].
Results
The laboratory bricks prepared in this way (KG 0 to KG 20) were tested to determine the material parameters apparent density, body apparent density, true density, porosity, body thermal conductivity, compressive strength and modulus of elasticity. From three or six individual values in each case, average values and standard deviations were calculated and compared with the results for the reference clay specimen without poreformers (KG 0) (» Table 1, 2).
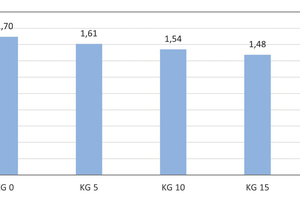
Development of the brick apparent density
With the addition of 5, 10, 15 and 20 wt% kieselguhr, the average brick apparent density falls from 1.70 g/cm3 of the reference specimen to 1.42 g/cm3 with the addition of 20 wt%. This corresponds to a 16.5-% reduction (» Fig. 6).
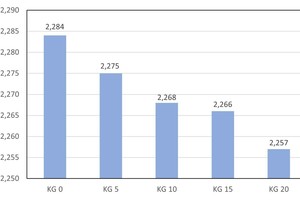
Development of the body apparent density
With the addition of 5, 10, 15 and 20 wt% kieselguhr, the average body apparent density falls from 2.284 g/cm3 of the reference specimen to 2.257 g/cm3 with the addition of 20 wt%. That corresponds to a reduction of 0.027 g/cm³ or 1.2 % (» Fig. 7). The kieselguhr effects microporosity of the body. A more pronounced macroporosification, as with the addition of polystyrene and saw dust, cannot be achieved with kieselguhr.
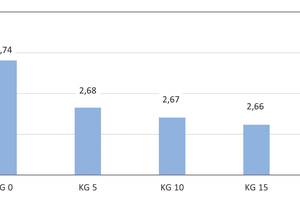
Development of the true density
The experimental determination of the pure densities was conducted on pulverized brick specimens with a helium gas pycnometer [14, 15]. The average values were calculated from three single determined values in each case (» Fig. 8). The brick reference specimens have an average true density of 2.74 g/cm3. With the addition of 5, 10 and 15 wt% kieselguhr, the true densities fall to 2.68, 2.67 and 2.66 g/cm3, respectively. The addition of 20 wt% kieselguhr leads to a reduction of the true density by 2.9 %. For the preparation of the true density measurements, the brick specimens were mechanically pulverized so that micropores existing in the body are destroyed and cannot exert any influence on the true density determined. For this reason, the very small decrease in the true density of just 2.9 % results.
Development of the body porosity
The curve of body porosity (» Fig. 9), i.e. the percentage of pore volume in the body volume, can be calculated based on the insertion of the apparent density and the true density in Equation (1) [14, 15]. Here, P stands for the body porosity (= porosity) in vol%, ρ for the true density in g/cm³ and ρR for the apparent density in g/cm³.
The reference brick specimens have an average body porosity of 38.08 vol%. With the addition of 5 wt% kieselguhr, the body porosity increases to 40.06 vol%. A further increase of the added kieselguhr to 10, 15 and 20 wt% leads to the increase in the body porosity to 42.29, 44.57 and 46.54 vol%, respectively. This corresponds to an increase of the body porosity by 22.2 % compared with the reference specimen.
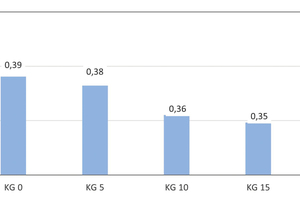
Development of the thermal conductivity λ10, tr.
Whereas the reference brick specimens without the addition of kieselguhr exhibit body thermal conductivity of λ10,tr. = 0.39 W/(m·K), increasing kieselguhr additions of 5, 10, 15 and 20 wt% led to a decrease to 0.38, 0.36, 035 and 0.33 W/(m·K), respectively. This corresponds to a reduction of 15.4 % compared with the reference specimen (» Fig. 10).
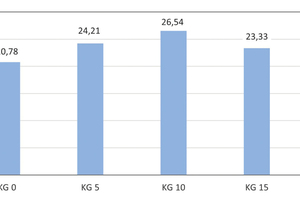
Development of the compressive strength
The reference brick specimens have an average compressive strength of 20.78 MPa. The addition of 5, 10, 15 and 20 wt% kieselguhr leads to an increase in the average compressive strengths to 24.21, 26.54, 23.33 and 23.05 MPa respectively. The maximum compressive strength of 26.54 MPa is achieved with the addition of 10 wt% kieselguhr. This corresponds to an improvement of the compressive strength by 27.7 %, although the porosity was increased by 11.1 %. The empirical standard deviations are integrated as error bars (» Fig. 11).
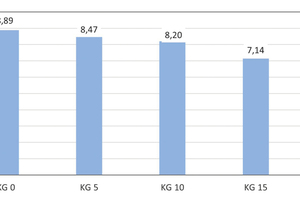
Development of the modulus of elasticity
The modulus of elasticity expresses the relation between stress and strain in the deformation of a solid body with linear-elastic behaviour. The modulus of the elasticity is greater, the more resistance a material shows to elastic deformation [15].
The reference brick specimens show an average value of 8.89 GPa for their modulus of elasticity. Rising kieselguhr additions of 5, 10, 15 and 20 wt% lead to a decrease in the modulus of elasticity to 8.47, 8.20 7.14 and 6.53 GPa, respectively (» Fig. 12). With an increase of the body porosity by 22.2 %, the resistance of the brick ceramic to elastic deformation decreased by 26.5 %.
The effect of the reduction of apparent density and body thermal conductivity with simultaneous increase of the brick compressive strength shown here is caused by the morphology of the diatoms in the ceramic matrix and has already been described as the “ceramic paradox” in relevant literature [4, 5]. To utilize this effect in the masonry brick industry, the diatoms have to survive temperature treatment in the tunnel kiln without reaching their melting point. From the literature data, it is know that the melting point of kieselguhr ranges from around 1 230 °C to 1 710 °C depending on the species and purity [16]. It can therefore be assumed that the morphology of the diatom scaffold would survive the tunnel kiln firing process with temperatures between 900 °C and 1000 °C for lightweight vertically perforated clay bricks. Only the outer areas of the diatoms are supposed to be sintered together with the surrounding brick matrix so that the high compressive strength of the diatoms can be transferred to the heavy clay ceramic.
There is theoretically the possibility that with suitable composition of the kieselguhr-containing clay mixes, eutectics form below the melting temperature of the pure brick clay. If this were to happen already in the temperature range of tunnel kiln firing from 900 to 1 000 °C, the structure of the diatoms can be broken down and the microporosity effect with simultaneous increase in the compressive strength rendered ineffective. To clarify this issue, tests were conducted on the respective melting onset of kieselguhr-containing clay formulations by means of simultaneous thermal analysis (STA) and hot stage microscopy.
Simultanaeous Thermal Analyses (STA)
To clarify the onset of melting of clay formulations containing kieselguhr, tests were conducted with Simultaneous Thermal Analysis (STA/DSC). For this purpose, in each case, 26 mg ± 2 mg of the specimens were presented in a platinum crucible and heated by means of STA within 4 h 42 min from room temperature to 1 440 °C and then treated for another hour at 1 440 °C. The gas atmosphere in the STA consisted of 80 vol% N2 and 20 vol% O2. An indication of the start of melting of the specimens is derived from the so-called onset temperatures, which result from the intersection of two tangents aligned to the curves as extrapolated temperatures. The onset temperatures mark the start of melting of a specimen. » Table 3 shows the determined onset temperatures Te [°C] for two measurements of the same specimens in each case as well as the calculated average values. From the study, it can be seen that the pure clay reference specimen exhibits the lowest melting start from 1 053.7 °C. The addition of 5 wt% kieselguhr to the clay formulation leads to a melting onset from 1 051.4 °C, i.e. practically to no significant change. Rising kieselguhr additions to 10, 15 and 20 wt% effect a successive increase of the respective melting start to 1 063.9 °C, 1 088.4 °C sowie 1 104.9 °C. The pure kieselguhr shows the highest melting start from 1 342.0 °C.
From the analysis, it can be seen that a lowering of the start of melting of clay formulation containing kieselguhr below the start of melting of the pure clay formulation without kieselguhr (reference specimen) caused by the formation of low-temperature-melting eutectics does not occur. The diatoms should therefore be able to survice the tunnel kiln firing process between 900 °C to 1 000 °C without structural change. In addition, the increasing addition of kieselguhr leads to a rise in the melting start above that of the pure clay reference specimen. It can be assumed that the diatoms do not change their morphology and fine structure up until their inherent melting start (in this case at 1 342 °C) and act as inorganic microporeformers.
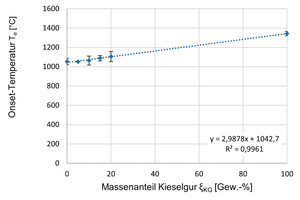
In the following » Fig. 13, the respective onset temperatures are shown graphically as a function of the kieselguhr addition. From the diagram, a largely linear dependence of the onset temperatures of the clay formulations on the kieselguhr addition can be identified. The higher the kieselguhr content in the clay formulation, the higher the resulting onset temperatures, that is the temperature of the respective onset of melting.
Analyses with the hot stage microscope
In the further course of the work, the thermal behaviour of the prepared clay formulations was also analysed with a hot-stage microscope. For this purpose, test specimens of kieselguhr-containing clay formulations were steadily heated to high temperatures in the measurement apparatus, which consists essentially of an oven, a light source and a microscope, steadily. During this temperature treatment, the test specimens were observed with a camera and any deformation and shrinkage of the test specimens were recorded and measured as shadow projections as a function of the temperature.
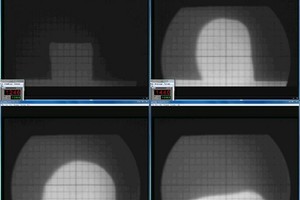
For the analyses, the previously prepared clay formulations with a content of 5, 10, 15 and 20 wt% kieselguhr were used. In addition, reference specimens of the clay formulation without the addition of kieselguhr and reference specimens of the pure kieselguhr, without the addition of a clay formulation, were prepared. The dry powder specimens were filled into the shaping tool of a toggle press and pressed to form quadratic-pyramidal test specimens with the dimensions 5 mm x 5 mm x 7 mm (L x B x H). These were placed in the hot stage microscope so that the lens of the microscope could observe the square base of the pyramidal test specimen. The test specimens were heated at a heating rate of 20 K/min from room temperature up to 1 500 °C. The recorded film with superimposed furnace temperatures enables evaluation of the test specimen’s deformation stages. From the so-called onset of softening (A), the spherical point (B), the hemisphere temperature (C) and the yield point (D), it is possible to analyse the influence of the temperature on the external specimen geomery and assess the softening and melting range (» Fig. 14) of the specimens [15].
The softening and melting ranges determined for the reference specimens and the clay specimens containing 5, 10, 15 and 20 wt% kieselguhr are given in » Table 4. For the pure clay formulation without the addition of kieselguhr (KG 0), a softening onset is shown at 1269 °C (A), the spherical point at 1347 °C (B), the hemisphere point at 1 414 °C (C) and the yield point at 1 511 °C (D). The onset of softening of the pure kieselguhr specimen (KG 100) is visible from the rounding of the edges of the observed surface from a temperature of 1 246 °C (» Fig. 14 A). At the spherical point at 1 440 °C (» Fig. 14 B), the projection is approximately spherical. From a temperature of 1 463 °C (» Fig. 14 C), the hemisphere point is reached, which marks the onset of the melting range of the pure kieselguhr. At a temperature of 1 527 °C, the yield point of the molten kieselguhr is reached (» Fig. 14. D»).
In the value comparison, it can be established that with the addition of the kieselguhr to the clay formulation, the temperatures of the softening and melting ranges are not lower in comparison with the pure clay formulation, but are observed to be in practically the same temperature intervals. Only the specimen for pure kieselguhr behaves differently. For this specimen, the end of the softening range is reached at 1 463 °C and the end of the melting range at 1 527 °C.
From the test, it can be derived that the determined softening and melting ranges of the clay and kieselguhr reference specimens as well as all kieselguhr-clay formulations lie well above the usual tunnel kiln sintering temperatures from 900 °C to 1 000 °C for lightweight vertically perforated bricks. Also from the analysis with the hot stage microscope, it can be derived that the morphology of the diatoms in the brick clay is preserved during tunnel kiln firing.
Between the results of the STA analysis and the analysis by means of hot stage microscopy, which were conducted on the same specimens, method-specific temperature discrepancies exost. These can be attributed to the fact that for the analyses with the STA, the dried clay reference specimen and the dried kieselguhr-containing clay specimens were initially crushed to a fine powder and then heated with a large surface area, while in the analysis with the hot stage microscope, small, but compact-pressed specimens were analysed.
Images with the scanning electron microscope (SEM)
To investigate the morphology of the diatom scaffold sintered with the brick clay at 900 °C and at 1 000 °C in an oxidizing firing, suitable brick specimens with a kieselguhr content of 20 wt% were analysed with a scanning electron microscope (SEM) (» Fig. 1-3). » Fig. 2, right, show the 5000 x magnification of a “honeycomb”-shaped diatom embedded and sintered in brick ceramic. It can be clearly seen that the shell of the diatom consisting of amorphous silicic acid (SiO2) is sintered with the remaining brick matrix and has survived the firing process at 900 °C very well. The inner diameter of the “honeycomb” shaped cavities measures around 3 µm. The morphological structure of the diatom scaffold was preserved under these firing conditions. » Fig. 3 shows diatoms embedded in brick ceramic at 5 000 x magnification. The firing temperature was 1 000 °C. At this firing temperature, too, the morphology of the diatoms was preserved.
Summary
From the study conducted, it can be concluded that the addition of moderate kieselguhr amounts to a marly clay formulation and oxidizing brick firing at 900 °C effects a reduction in the apparent density and the body thermal conductivity with a simultaneous increase in the compressive strength and porosity. The addition of 20 wt% kieselguhr led to a lowering of the brick apparent density by 16.5 % and to a decrease in the body apparent density by 1.2 %. At the same time, the body thermal conductivity was reduced by 15.4 % with simultaneous increase in the compressive strength by 10.9 %. With the addition of 10 wt% kieselguhr, the compressive strength reaches a maxumum increase of 27.7 %. At the same time, the apparent density is lowered by 9.4 % and the thermal conductivity λ10,tr by 7.7 %.
With this investigation, the “ceramic paradox” occurring with the use of kieselguhr in heavy clay ceramics as known from the relevant literature could be reconfirmed. This effect is caused by the particular morphology of the diatoms in the brick matrix. During the ceramic firing process, the high-strength shells of these diatoms (minimalist biological design) sinter together with the remaining brick ceramic. As a result the high strength of the diatoms is transferred to the surrounding brick ceramic, lead to an increase in its compressive strength. The cavities inside the diatoms lead to microporosity of the body. As a result, the apparent density and the body thermal conductivity decrease. This effect is only realized when the morphology of the diatom structures is not destroyed by the temperatures between 900 °C and 1 000 °C prevailing during firing in the tunnel kiln.
In this work, it was established that the onset of melting of the marly clay reference specimen and the marly clay mix with 5 wt% kieselguhr takes place at temperatures of around 1 050 °C. The futher increase in the kieselguhr addition to 10, 15 and 20 wt% leads to a further successive increase in the temperatures of the melting onset to approx. 1 064 °C, approx. 1 088 °C and approx. 1 105 °C. The pure kieselguhr shows a melting onset only at 1 342 °C. At all lower temperatures, the melting temperature of the ceramic matrix is achieved, but the melting temperature of the kieselguhr is not reached. Only when this temperature is reached is the microstructure of the diatoms lost. These high temperatures are not reached in tunnel kilns for firing vertically perforated bricks with high thermal insulation, so that the effect of the “ceramic paradox” should be realized.
In the course of the investigations, no eutectics caused by the kieselguhr were detected between 900 °C and 1 000 °C. The SEM analyses of the kieselguhr-containing marly clay specimens fired at 900 °C (» Fig. 1, 2) and 1 000 °C (» Fig. 3) show that in these firing conditions the morphology of the diatoma scaffold is preserved and microporositiy can be effected. The pore sizes that can be obtained with kieselguhr are in the micrometre range. As a result, the achievable porosity effect cannot be in the same order of magnitude as that obtained with macroscopic poreformers like sawdust and polystyrene.

![» Fig. 1: SEM images of diatom fragments in brick ceramic made of marly clay with 2 000x magnification. The firing temperature was 900 °C. The morphology of the diatoms is preserved [6]](https://www.zi-online.info/imgs/1/9/6/0/2/8/5/tok_cab9747f0409092b7e1fc1c8b318cdcd/w300_h200_x600_y224_Abb._1_neu-a141b53c30bcc1b7.jpeg)
![» Fig. 2: SEM images of diatoms in brick ceramics made of marly clay with 5 000x magnification. The firing temperature was 900 °C. Left: Morphology and fine structure of the diatom scaffold were preserved. Right: Honeycomb diatom is sintered together with the surrounding brick ceramic [6]](https://www.zi-online.info/imgs/1/9/6/0/2/8/5/tok_c948eab668c52c9321e21b274063c932/w300_h200_x600_y230_Abb._2_neu-f300e649f57506f0.jpeg)



![» Fig. 9: Increase of the body porosity (pore volume) [vol%] of laboratory bricks with increasing of addition of kieselguhr of 5, 10, 15 and 20 wt% to the clay formulation in comparison with the reference specimen without the addition of kieselguhr. Numbers in German notation](https://www.zi-online.info/imgs/1/9/6/0/2/8/5/tok_7817a03f811ae5cef4e92422db2ccc80/w300_h200_x600_y245_Bild9-464f8130ef06e8e9.jpeg)