Time evolution of the mineral carbonation of ceramic bricks in a simulated pilot plant using a common clay as sealing material at superficial conditions
Mineral carbonation, or mineral sequestration, of CO2 was proposed by Seiftriz in 1990 [3] as an equivalent process that occurs naturally from weathering of silicate rocks into carbonates in a CO2-rich environment. However, these natural processes take place on the geological scale of time. Of the alkaline earth metals, calcium and magnesium are by far the most common in nature. Large quantities of industrial minerals and industrial wastes rich in alkaline oxides are available for CO2 fixation. Therefore, calcium and magnesium are generally selected for the sequestration of mineral CO2 . (Extract from the doctoral thesis [1,2] by Domingo Martín)
The carbonation reactions of calcium and magnesium oxides are strongly exothermic as the carbonate state is a low carbon energy state [4]:
CaO (s) + CO2 (g) → CaCO3 (s) (ΔHr = -179kJ/mol)⇥Eq. 1
MgO (s) + CO2 (g) → MgCO3 (s) (ΔHr = -118kJ/mol)⇥Eq. 2
However, the most common form in nature of calcium and magnesium is in the form of silicate minerals versus the binary oxides of these elements. These silicates are capable of being carbonate since the silica present in these minerals is easily interchangeable with carbonate ions. In general, the silicates reaction is [5]:
(Mg, Ca)x SiyOx+2y+z H2z (s) + xCO2 (g) → x (Mg, Ca)CO3 (s) + ySiO2 (s) + zH2O (l)⇥Eq. 3
These reactions are still exothermic, but to a lesser extent than carbonations of pure oxides. But as stated above, these naturally occurring processes require long periods of time.
That is why many investigations have gone towards accelerating this procedure. The wet route is one of the acceleration techniques since the presence of water favours the formation of carbonic acid through the dissolution of CO2 that allows the extraction of the reagent, alkaline-terrestrial ions, from the matrix for subsequent fixed and stable carbonation.
The mechanisms of carbonation of minerals in a wet process can be summarized in the following steps [6]:
1. CO2 dissolution in aqueous phase:
CO2 (g) + H2O (l) → H2CO3 (g)⇥Eq. 4
H2CO3 (aq) → H+ (aq) + HCO3- (aq)⇥Eq. 5
HCO3- (aq) → H+ (aq) + CO32- (aq)⇥Eq. 6
2. The leaching of divalent ions from the mineral matrix:
(Ca, Mg)-silicate (s) + 2H+ (aq) → (Ca, Mg)2+ (aq) + SiO2 (s) +
H2O (l)⇥Eq. 7
3. Carbonate precipitation:
(Ca, Mg)2+ (aq) + HCO3- (aq) → (Ca, Mg)CO3 (s) + H+ (aq)⇥Eq. 8
or
(Ca, Mg)2+ (aq) + CO32- (aq) → (Ca, Mg)CO3 (s)⇥Eq. 9
The main aim of this study was to investigate the process of the mineral carbonation by the wet route of a type of brick used in construction. This brick was selected for its chemical and mineral composition. The carbonation efficiency of the selected brick, the AUC1, was determined among other bricks on a smaller scale, laboratory scale [7]. The ceramic brick was mainly composed of quartz, diopside, wollastonite and orthoclase and minor anhydrite.
Investigate the process of mineral carbonation
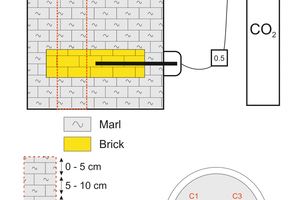
The carbonation process was carried out in a specially designed reaction chamber (»Fig. 1) in which the recovery of a disused clay quarry filled with these bricks was simulated and CO2 was injected for sequestration under conditions of ambient temperature and low pressure. A common clay was used as sealing material during the carbonation test contains calcite, quartz, illite, smectite and kaolinite and trace of dolomite.
The reaction conditions were the following: 0.5 bar constant pressure controlled by a mass flow controller, 4:1 solid/water ratio and room temperature. And the reaction time was variable between 5 months to 12 months (5, 7, 9 and 12 months). After each period, a sampling was drawn and the core obtained was cut in sections of five-centimetre thickness. The first five sections from the top corresponded to the upper clay layer, the next was the crushed brick, and the last corresponded again to the marl at the bottom.
Both raw brick and clay were characterized, and the evaluation of the interaction of the CO2 gas flow in the different sectioned layers was analyzed for each carbonation test. Different characterization techniques were employed, such as XRF, XRD, SEM-EDS, DTA-TG, C-elemental, N2- and CO2-BET, Hg-porosimeter, and IPC-EOS [8].
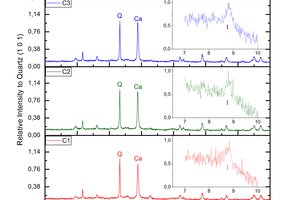
Calcite (calcium carbonate) as a carbonated mineral in brick is evident, as well as the destruction of calcium silicate (wollastonite and diopside), anhydrite and orthoclase (potassium feldspar). Besides, minor dolomite (calcium and magnesium carbonate) precipitated and some illite (mica group) formed probably due to orthoclase alteration (»Fig. 2).
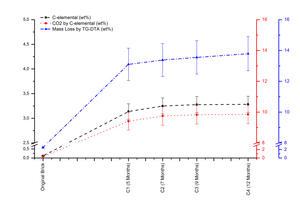
According to the C-elemental and mass loss by TG-DTA results of carbonated samples (»Fig. 3) confirms that the process converts the gaseous CO2 to solid calcium carbonate revealed in X-ray patterns.
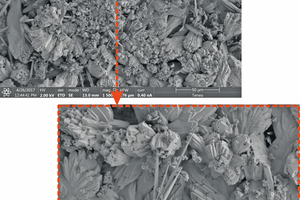
The morphological analysis of the porosity in the brick grains showed an increase in the macro-porosity due to the destruction of silicates on the surface. Whereas the micro-porosity decreases due to the growth of new calcite reducing the size of the micro-pore to nano-pore, more evident in the first carbonation tests. In addition to the chemical retention by carbonation of CO2, the absorption isotherms showed physical retention of the gas.
Alternative to carbon capture and storage (CCS)
technology
The layers of clays used as sealing material, the calcium carbonate content increases indicating a higher percentage of CO2 retained. Broadly, the total calcium and carbon increased, especially in contact with the brick layer, due to a higher quantity of calcium in the marl. Such increases of carbonate and fluid would be related to the possible transfer of CO2 on the water and with an external contribution of calcium from the bricks. Additionally, CO2-BET and CO2 adsorption showed physical retention in micro- and nano-pores and also the carbonate precipitation covering/filling pores.
As a conclusion, it is possible to indicate that during this research acceptable levels of carbonation have been archived under favourable conditions of low pressure and room temperature with low energy costs and, therefore, environmental protection. The system designed could be extrapolated as an alternative to carbon capture and storage (CCS) technology to a real case of a quarry recovery with ceramic brick waste as a host material for mineral carbonation by direct injection of a CO2 and using common clay as sealing materials.
[† Deceased on 29th January 2019]