Decarbonization: Using Hydrogen and Ammonia in the Clay Brick and Tile Industry
The clay brick and tile industry is facing the challenge of significantly reducing its CO2 emissions to achieve long-term climate goals. In this paper, we show that hydrogen and ammonia can be used in the industry as alternative firing gases without any adverse effect on the quality or visual appearance of products. With regard to ammonia, questions remain concerning the realization and adaption to the firing process in the tunnel kiln, so that further specification is needed. The key to wider realization is the future availability and economic efficiency of the gases.
1. Introduction
The transition to a greenhouse-gas-neutral industry is a principal component of global efforts to contain climate change. Especially in energy-intensive industries like the clay brick and tile industry, there is an urgent need for the substitution of fossil fuels with climate-friendly alternatives. Against this background, research projects have been initiated in recent years to develop and trial suitable technological solutions. For example, the H2 Brick project [1] has already been successfully concluded. The follow-up project NH3 Brick is currently in its final phase. Both projects are pursuing the goal of systematically investigating and evaluating the potential use of hydrogen and ammonia, both in their pure form as well as mixed with natural gas, as alternative firing gases for high-temperature processes in clay brick and roofing tile production. The effects on the combustion behaviour, the quality of the brick products and the emission characteristics are the focus of the experimental and numerical investigations.
Against the background of achieving global and national climate goals, the decarbonization of industrial processes is coming to the fore. In Germany alone, the clay brick and tile industry emitted around 1.74 mill. tonnes CO2 in 2020, of which around 88 % can be attributed to the use of natural gas [2]. On European scale, the ceramics industry causes the emission of around 19 mill. tonnes CO2 annually, around 1 % of the total industrial emissions in Europe. The substitution of natural gas with renewable fuels can therefore make a significant contribution to lowering emissions.
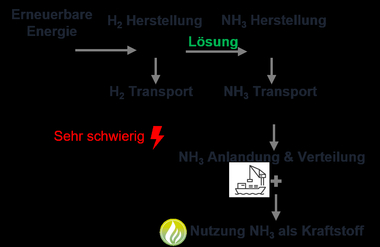
With the development of renewable energies, green electric energy is increasingly available worldwide, which can be transformed into chemical energy sources like hydrogen or ammonia by means of power-to-gas technologies. Green ammonia, which is produced based on the synthesis of hydrogen (from electrolysis with renewable power) and nitrogen (from air), offers considerable advantages over hydrogen with regard to storage and transport. While hydrogen can only be liquified at - 252 °C, the liquefaction of ammonia is possible already at – 33 °C under normal pressure or at 20 °C under a pressure of 9 bar.
However, the use of hydrogen and ammonia as firing gas in the clay brick and tile industry also comes with technical challenges. The changed combustion behaviour influences the firing and control system as well as the kiln atmosphere, which in turn can impact the product quality. In this context, the Institut für Ziegelforschung Essen e.V. (Brick and Tile Research Institute Essen Regd. or IZF) in cooperation with the Gas- und Wärme-Institut Essen e.V. (Gas and Thermal Energy Institute Essen Regd. or GWI) is studying the technical and emission-relevant impact of ammonia combustion under near-field conditions in the scope of the research project “Ammonia as a renewable fuel in the clay brick and tile industry”. The goal is to prove the feasibility of a largely CO2-neutral brick production based on the use of renewable fuels like ammonia and support the industry on its technological path to climate neutrality by 2050.
2. Experimental and numerical studies
The goal of this phase was to closely analyse and evaluate the combustion behaviour of ammonia in near-field conditions.
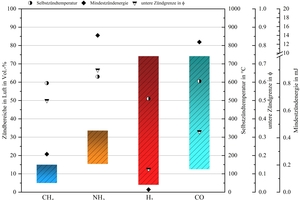
As the comparison with other firing gases in »Fig. 1 shows, ammonia possesses high ignition energy and a narrow ignitable range, while exhibiting very low rates of combustion. These are properties that make stable flame control difficult. To improve flame stability, two approaches were trialled: on the one hand chemical stabilization based on the mixing in of reactive gases like methane (CH4) or hydrogen (H2), and on the other hand a design measure based on targeted vortexing in the oxidator stream.
In the first experimental studies, two approaches for stabilization of the flame with the use of ammonia as fuel were systematically investigated. On the one hand, chemical flame stabilization with the addition of reactive gases like methane (CH4) or hydrogen (H2), and on the other hand design-based flame stabilization with targeted vortexing of the oxidator stream at the burner entry.
For this purpose, a modular test burner was developed that permits variable vortexing and a graduated air supply. This enabled systematic study of the flame behaviour for different mixing ratios.
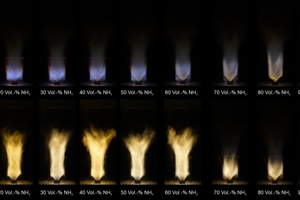
The results are shown in »Fig. 2. Already low additions of NH3 to methane lead to a visible change in the flame colour, from blue to yellowish orange, which can be attributed to changed emissions of reactive combustion products. For H2-NH3 mixes, ammonia also makes itself noticeable. While the pure hydrogen flame remains almost invisible, it becomes visibly recognizable with the addition of NH3.
Particularly striking is an abrupt change in the flame pattern for an ammonia content between 60 and 70 vol%. This transition can presumably be attributed to the change in the dominant stabilization mechanization, from chemical to geometrically induced stabilization by means of burner flame vortexing.
For the experimental studies on semi-industrial scale, two lance burner systems widely used in the brick and tile industry are studied; these systems were originally designed for operation with natural gas. First tests were conducted with a burner output of 80 kW and show that a complete combustion of 100 % ammonia is technically realizable under certain boundary conditions. In the experiments we conducted, the test kiln was first heated with natural gas to a target temperature of 1 050 °C before ammonia was gradually added to the firing gas (first series of tests with natural gas, second series of tests with hydrogen) until full substitution was achieved.
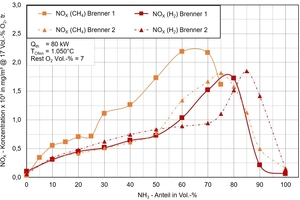
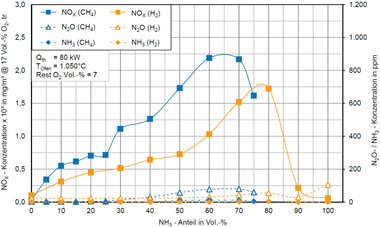
The emissions of nitrogen oxides (NOx) show a clear dependence on the geometry of the burner used. Despite this sensitivity, with proven reduction measures, moderate emission values can be achieved overall. The measurement results shown in »Fig. 3 illustrate the NOx-emission behaviour for various gas mixes in which ammonia was gradually mixed in 10 vol% steps into either natural gas or hydrogen.
The results with the conventional burner systems from the clay brick and tile industry show a characteristic curve. With increasing ammonia content, the NOx emissions first increase and reach with a content of 60 to 70 vol% a maximum of around 2 250 mg/m³ (relative to 17 % oxygen). Above that, the emission value decreases again, which led to similar findings in different burner tests.
These results clearly show that the emission behaviour is influenced not only by the ammonia content, but also by the carrier gas used and the burner design. This is a key aspect for the future use of ammonia in industrial high-temperature processes in the clay brick and tile industry.
Numerical flow simulations by means of computational fluid dynamics (CFD) were conducted to evaluate in detail the suitability of conventional burner systems for ammonia combustion.
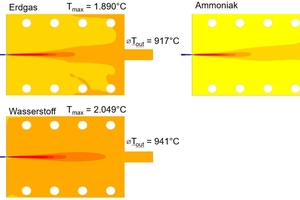
In »Fig. 4, the simulation results for the temperature distributions in the test kiln with the use of various firing gases are shown (for an air/fuel ratio of λ = 1.5). It can be clearly seen that for the combustion of hydrogen the highest maximum temperatures are reached, with a peak value of 2 049 °C. This is well above the values obtained with natural gas and in particular with ammonia. The differences can be attributed essentially to the specific adiabatic flame temperature of the fuels used. For hydrogen this is 2 106 °C, for natural gas 1 951 °C and for ammonia just 1 798 °C.
Against this background, the central question is whether the comparatively low adiabatic flame temperature of ammonia is sufficient to reach and stably maintain the process temperature necessary in brick production. This question is especially relevant in respect of the full substitution of fossil fuels with ammonia and is specifically addressed in further studies.
3. Firing tests with test specimens
Following comprehensive analysis of alternative firing gases for the decarbonization of the clay brick and tile industry, the focus is now shifted to an analysis of its effects on product quality, especially in respect of colour changes and structural properties of the fired brick products. As different firing gases lead to widely deviating kiln atmospheres, a systematic evaluation of the resulting material properties is essential.
For specific study of the effects of ammonia, four firing gas configurations were defined:
100 % natural gas (reference firing),
a mix of 50 % natural gas and 50 % ammonia by volume,
a mix of 50 % hydrogen and 50 % ammonia by volume as well as
100 % ammonia.
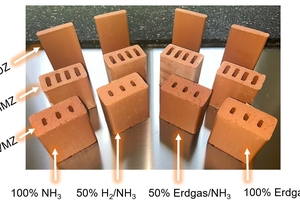
On the basis of these scenarios, model bricks were prepared for various applications (facing bricks, clay blocks and roofing tiles). The firing processes were then conducted in a test kiln, with each of the above-mentioned gas configurations being studied separately. The goal is to reproducibly record and evaluate the influences of the different fuels on colour, homogeneity, and technical quality of the brick products.
On account of the different firing curves of the product types used, the firing setting is divided into two separate configurations. In addition to the brick products described, round bars are also fired, which are used for determination of the flexural strength.
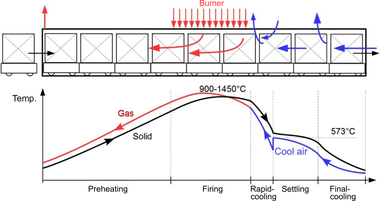
The facing bricks (VMZ) and the roofing tiles (DZ) reach maximum temperatures of around 1 050 °C in the firing zone. During the cooling phase, the temperature is held for a short time in the range of the quartz inversion (around 573 °C). In comparison, the clay blocks (HMZ) have a much shorter firing time. In addition, the maximum firing temperature here is around 950 °C. For this product type, the focus is on the requirements for density and compressive strength.
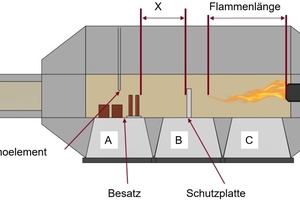
The test kiln in which the firings were conducted is shown in »Fig. 5. On account of the limited area of the specimen support, the test specimens were positioned such that a very uniform flow and temperature distribution around each individual specimen is ensured. The maximum height of the firing setting is limited by the thermocouple installed in the kiln ceiling, as a result of which a maximum setting height of around 170 mm can be reached. The test kiln has three specimen supports, indicated with A, B and C. The specimen supports B and C are not suitable for placing of the test specimens on account of the direct flame effect. To protect the specimens against direct flame contact, a ceramic shielding plate was installed on specimen support B. At the same time, this is used to ensure a homogeneous temperature distribution in the kiln chamber while the actual firing specimens were positioned on specimen support A.
4. Effects on product quality
All brick product specimens produced in the tests could be successfully fired with the use of hydrogen and ammonia in the test kiln shown in »Fig. 5. The product-specific firing curves for the different specimens were set with only minor deviations. During the high-temperature phase, the average oxygen content in the firing chamber was around 7 %, which is of key importance in respect of the product colour, as the oxygen crucially influences the oxidation processes during the firing.
In »Fig. 6, it can be clearly seen that the different kiln atmosphere, caused by the use of hydrogen and ammonia mixes, does not have any negative effects on the colour of the finished products. This applies both to the fair-face brick products such as the facing bricks and roofing tiles (VMZ and DZ) as well as to the clay blocks (HMZ) for which a reddish colour is still expected despite the lower optical relevance. Colour measurements with a spectral photometer confirmed merely minimal deviations from the natural gas reference, which were in the region of the usual batch-related colour variations. A systematic correlation between the firing gas used and colour change could not be proven.
Also, in respect of key firing-related properties like firing shrinkage and firing loss, no significant differences are observed between the individual firing gas configurations. The firing shrinkage, that is the volume-related change in the dimensions of the specimen as a result of mineralogical restructuring and sintering processes, depends primarily on the clay material used and was maintained at the product-typical level throughout all the tests. As expected, the clay blocks exhibit stronger firing shrinkage as in addition to the mineral transformation, organic pore-formers burn out and contribute to volume reduction. Moreover, the firing losses, defined as the mass loss caused by the burn-out of volatile constituents, remain within the usual ranges.
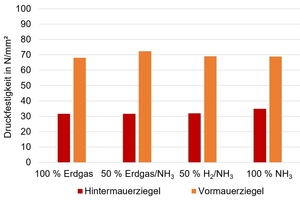
For the evaluation of the material quality, various relevant characteristics were determined. For clay blocks, the focus was especially on the brick density and compressive strength. The targeted values of at least 750 – 800 kg/m³ for the density and ≥ 12.5 N/mm² for the compressive strength were achieved in all cases. The compressive strength of the fired specimens for clay blocks and facing bricks are shown in »Fig. 7. Key feature of fair-face masonry products like facing bricks and clinker bricks is higher sintering on account of higher firing temperatures. This leads to increased water repellence and lies typically in the density range from 2 000 – 2 500 kg/m³ or more. Accordingly, the compressive strengths of these products lie much higher at ≥ 35 N/mm².
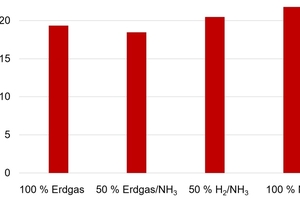
Roofing tiles also exhibit densities in the range of 2 000 kg/m³ and must on account of their exposed position demonstrate high frost resistance and low water absorption. In addition, for this product group, the flexural strength is particularly important. Here, in all tested cases, the target value of at least 15 N/mm² in the fired state can be reliably achieved (»Fig. 8).
Overall, the results show that neither the colour nor the mechanical and physical properties of the products were adversely impacted by the use of hydrogen or ammonia fuels. All values measured range within the tolerances usual for industrial production. A direct dependence between gas mix and brick material properties could not be determined. The tests therefore confirm the unreserved suitability of alternative firing gases for use in brick production, both in view of processing and quality aspects.
5. Summary and outlook
The investigations presented show impressively that the use of hydrogen and ammonia as alternative firing gases in the clay brick and tile industry is technically feasible. Both on laboratory scale as well as in semi-industrial conditions, stable combustion processes could be realized and the required process temperatures reliably reached. The tests conducted clearly show that existing burner systems are in principle capable of running on these alternative fuels, although targeted design optimizations could further improve the efficiency and emission characteristics.
With regard to safety aspects, no abnormalities could be determined. Operation with hydrogen and ammonia ran trouble-free, as a result of which it becomes clear that with proper use and corresponding plant equipment no additional risks result. Verification for the use of these firing gases in the tunnel kiln has not yet been provided. With hydrogen, already partial substitutions have been realized. For the use of ammonia, the safety-relevant challenges are much greater, on account of the toxicity alone. The ammonia slip and the nitrous oxide (laughing gas) emissions must be considered further and require a detailed study of the tunnel kiln concept.
Especially notable is that the brick products fired with alternative firing gases do not show any significant differences in respect of quality nor visual features nor colour in comparison with natural gas combustion. Accordingly, the product integrity is guaranteed with the use of CO2-neutral gases.
The crucial challenge lies therefore no longer exclusively in proving technical feasibility, but in the wide industrial availability and economical scalability of these gases. At present, both hydrogen and ammonia are not sufficiently scaled in respect of availability, infrastructure, and price for comprehensive supply of an energy-intensive industry like clay brick and tile production.
So that the contribution of the clay brick and tile industry to the national and European climate strategy, especially to the aspired greenhouse-gas neutrality by 2045 and 2050, can be realized, a significant increase in the production of green hydrogen and ammonia is needed. This must be supported by corresponding programmes to promote an increase, the regulatory framework, and the development of logistical infrastructures. Only in this way can a stable supply at competitive prices be guaranteed in the long term and a viable decarbonization strategy established for the entire industry.
Overall, the results show clearly that the decarbonization of the clay brick and tile industry is certainly not some far-off future scenario, but considerable efforts are still needed to achieve technical realization. It will be crucial to create the political, infrastructural and economic conditions so that these technologies find their way from trial operation into widespread industrial practice. Only then can the industry fully develop its contribution to a climate-neutral future.
The project (NH3 Bricks) was funded by Germany’s Federal Ministry for Economic Affairs and Energy via the Industrial Collective Research (IGF), Project Number 22893.